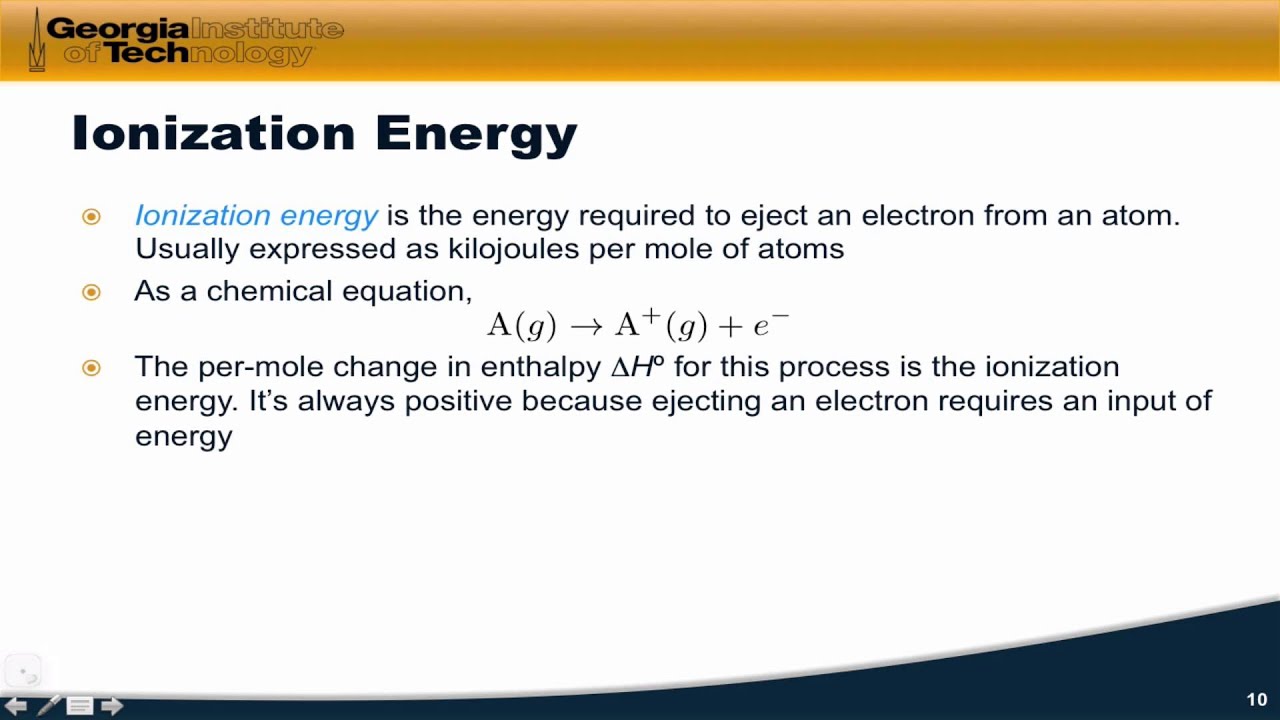
How To Identify Ionization Energy
7.4: ionization energy Ionization energy atomic first periodic table number elements radius group trends variation chemistry highest ion vs energies lowest general plot Ionization energy first energies elements electron element atom definition removed
7.4: Ionization Energy - Chemistry LibreTexts
Ionization energy table periodic elements chemistry first has electron energies higher general principles lower does why than formation ion properties First ionization energy trend exceptions Ionization element periodic successive
Ionization energy
Ionization energy periodic atoms elements table ppt powerpoint presentationIonization energy Which following pairs of atoms, have a lower electron affinity? a) ca,kPeriodic properties of the elements.
Ionization energyIonization elements energy energies periodic trends group main first 20 atomic properties table second electron successive atoms configuration general plot Graph energy ionization periodic trends elements table chemistry showing hydrogen argon chemwiki properties figurePeriodic table with ionization energy values (labeled image).

Ionization energy first periodic table elements trends chemistry sandbox user nuclear charge wikieducator mol kj showing electron affinity worksheet
Periodicity ionization periodic electron affinity electronegativity radius atomic ionic sciencenotes repeating refers such02.02 periodicity and ionization energy Ionization periodic labeledPeriodic properties of the elements.
What is ionization energy? definition and trendSavvy-chemist: ionization energy (1) definition and how its measured Energy ionization atom hydrogen state ground spectrum its calculate chemist lines do equation will which savvy only partIonization energy tend to increase from left to right in a periodic.
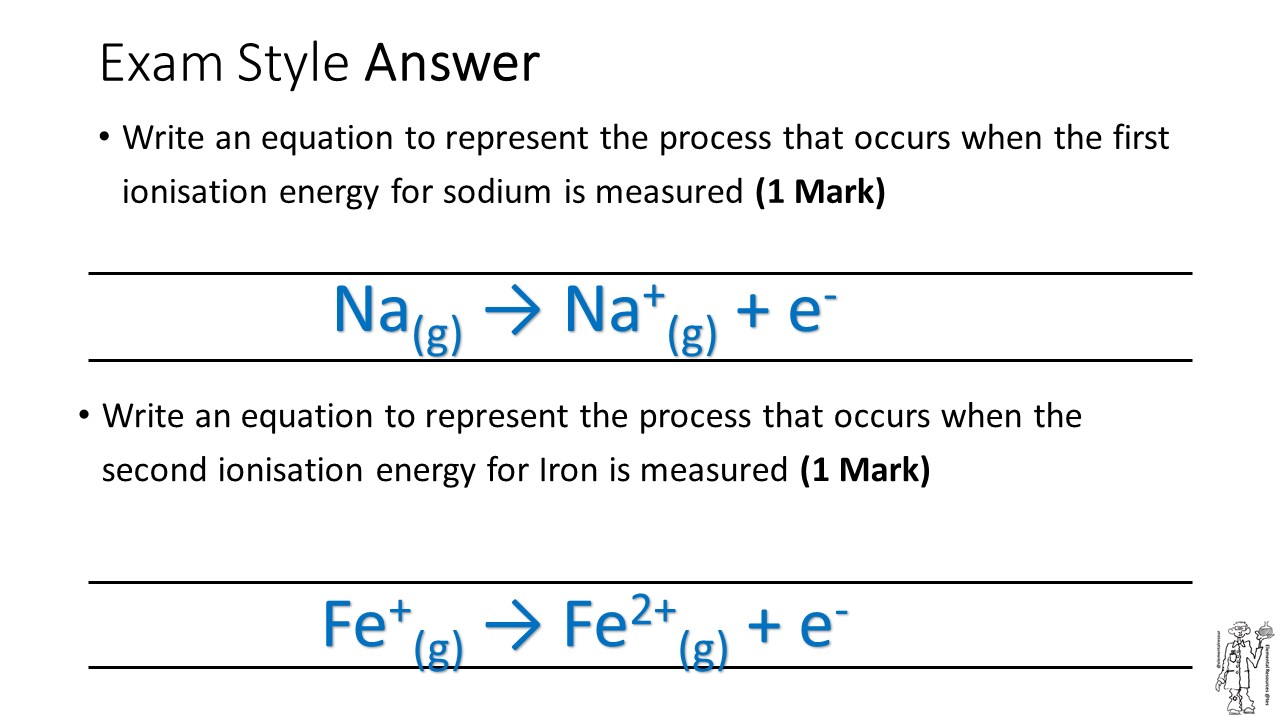
Ionization successive energies
Ionization energy values periodic tableUser:phaello/sandbox/chemistry/ionization energy Ionization energy atoms periodic ppt first group why across energies table trends general elements powerpoint presentation increasesIonisation docx.
Ionization energy ionic compound ions ppt powerpoint presentation remove electron eachPeriodic table trends ionization energy electronegativity Ionization energy first factors affect sizeIonization periodic enthalpy electronegativity electron.

Which of the following has highest first ionization energy? 1)sc 2)k
7.4: ionization energyPeriodic trends Ionization energy basicIonisation energy as level.
Successive ionization energiesIonization energies electron affinities libretexts periodic metals exceptions bonding chem Ionization elements first energies periodic energy properties element highest has which graph group chemistry table period atoms trends main halfIonization energy.

Ionization energy
Quizizz ionizationIonization highest periodic socratic Electron affinity periodic radius elements atom atoms ion socratic increases magnesium decreases column compareIonization energy group table chemistry period increases.
Suka chemistry: factors that affect the size of the first ionization energy .