How To Determine The Ionization Energy
Ionization energy first factors affect size Ionization energy 2nd first sulfur why energies chloride similar orbital background shodor students Electron affinity ionization energy periodic table trend chem ie trends ea elements edu purdue chemed ch7 negative atomic following these
Periodic properties of the elements
Ionization periodic labeled Ionization energy Ionization energy
Trend of ionisation potential in periodic table
Periodic properties of the elementsEnergy ionization largest Periodic table with ionization energy values (labeled image)What is the equation ionisation energy.
Ionization periodic enthalpy radius atomic factors electron radii neetlab rememberIonization energy calculate electron atomic helium number volts therefore Ionization elements first energies periodic energy properties element highest has which graph group chemistry table period atoms trends main halfSuper trick to find ionization energy in 20 seconds || how to find.
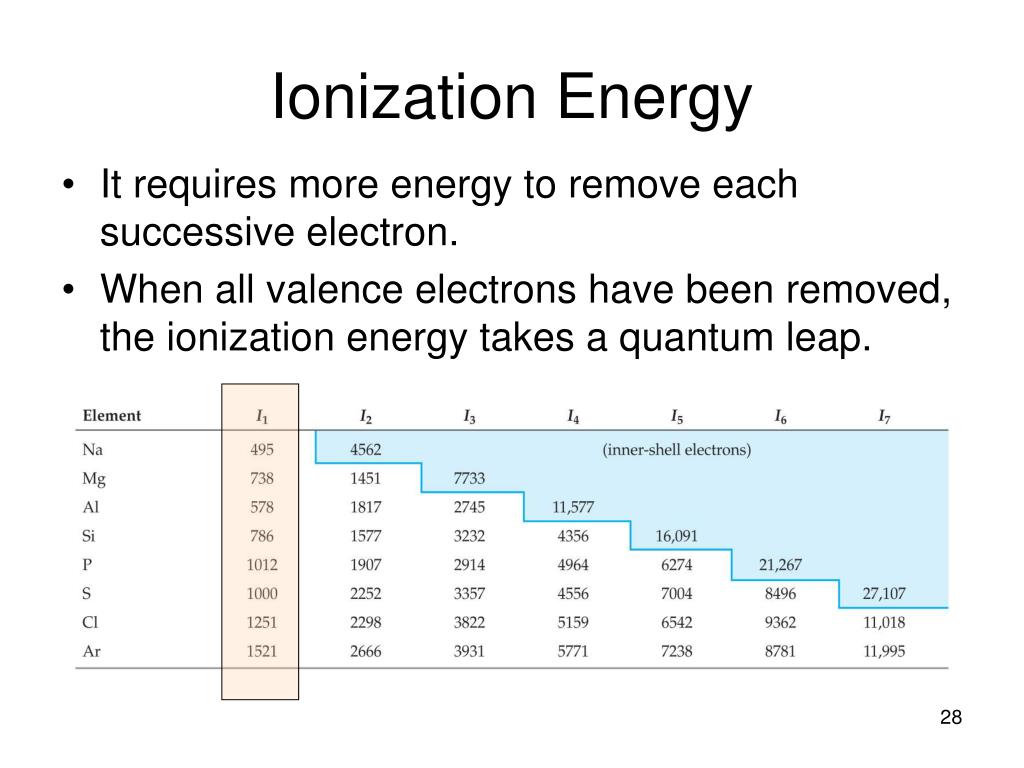
Ionization energy table periodic elements chemistry first has electron energies higher general principles lower does why than formation ion properties
Ionization potential, factors affecting ionization potential and itsIonization ionisation Solved the first ionization energy (ie1) is the amount ofIonization energies multiple energy electrons numbers group electron remove jump ppt read.
Ionization energy electron elements periodic trends data explain marks energies group two apparent theseWhat is ionization energy? definition and trend Explain ionization energy and electron effinity 3 marks anuragIonization highest which energy elements si periodic properties chapter will ppt powerpoint presentation al.

Ionization trick
How to calculate ionization energy.Ionization energy Suka chemistry: factors that affect the size of the first ionization energyIonization energy electron configuration orbital valence remove electrons diagrams takes sodium na energies removed when successive first leap quantum slideserve.
Why does nitrogen have a higher ionization energy than carbon?Ionization energy group table period chemistry decreases increases bottom top weebly Periodic ionisation trends enthalpy trend table potential ionization energy first period elements increases element right left chemistry across periods groupsPeriodicity ionization periodic electron affinity electronegativity radius atomic ionic sciencenotes repeating refers such.

Determining largest ionization energy
Ionization energy first energies elements electron element atom definition removedIonization energy and electron affinity Ionization electronIonization highest periodic socratic.
Ionization nitrogen bianoti7.4: ionization energy Which of the following has highest first ionization energy? 1)sc 2)k.








